Main components of lead sulfate battery include positive electrode, negative electrode, electrolyte, separator and battery shell. The positive electrode is mainly composed of lead dioxide (PbO2), which is brown and black. The negative electrode is composed of spongy lead (Pb); The electrolyte is a dilute sulfuric acid (H2SO4) solution; The separator is usually made of a non-conductive microporous material, its role is to prevent direct contact between the positive and negative electrodes resulting in short circuit, while allowing ions in the electrolyte to pass freely; The battery housing is usually made of acid-resistant plastic or other non-conductive materials to ensure mechanical strength and tightness. In these basic components, the concentration of electrolyte, temperature and the quality of positive and negative electrode materials will directly affect the performance and life of the battery.
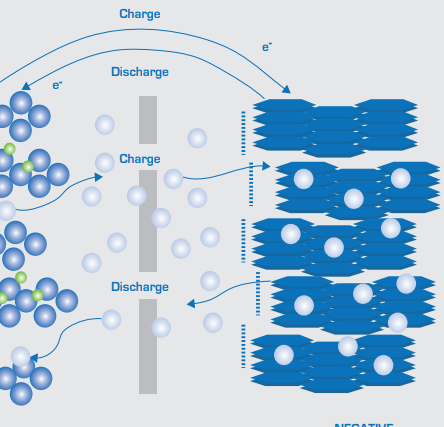
the battery discharge is a chemical reaction process, in this process, the chemical energy into electricity. When discharged, the positive lead dioxide reacts with sulfuric acid in the electrolyte to form lead sulfate (PbSO4) and water (H2O). At the same time, the spongiform lead of the negative electrode also reacts with sulfuric acid to form lead sulfate and water, which can dilute the electrolyte, resulting in a decrease in the concentration of the electrolyte. The chemical equation of the discharge reaction can be expressed as:
negative reaction: Pb + HSO4- → PbSO4 + H+ + 2e-
Positive reaction: PbO2 + HSO4- + 3H+ + 2e- → PbSO4 + 2H2O
Total reaction: Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
In the discharge process, due to the reaction of lead dioxide and spongy lead to the same lead sulfate, which will lead to a gradual reduction of positive and negative electrode active substances, the battery discharge capacity will decrease until the battery active substances are exhausted. The battery discharge ends. Procedure The electrons released between the two poles of the battery flow through an external loop to supply the equipment that needs power.
The charging process is essentially a reverse reaction of the discharge process, that is, by applying an external voltage to the battery, forcing the current to flow backward to re-convert chemical energy. During charging, a reduction reaction occurs in the positive electrode, reducing lead sulfate to lead dioxide; The negative electrode is oxidized, reducing lead sulfate to spongiform lead, and producing sulfuric acid. The chemical equation of the reaction during charging is the opposite of that of the discharge reaction:
Negative reaction: PbSO4 + H+ + 2e- → Pb + HSO4-
Positive reaction: PbSO4 + 2H2O → PbO2 + HSO4- + 3H+ + 2e-
Total reaction: 2PbSO4 + 2H2O → Pb + PbO2 + 2H2SO4
During the charging process, because the lead sulfate is converted back to the original positive and negative electrode materials, the chemical structure of the battery is re-established, and the battery can be reactivated. With the progress of charging, the concentration of sulfuric acid in the electrolyte gradually increases, the internal resistance of the battery begins to decrease, and the battery capacity gradually recovers. Under normal circumstances, to fully complete the charging reaction, the battery needs to be charged above its nominal voltage to ensure full, and the charging rate needs to be controlled to prevent the electrolyte from overheating, positive corrosion, or hydrolysis to produce oxygen and hydrogen resulting in safety issues.
It is worth noting that during the charge and discharge process, lead sulfate batteries will produce a certain degree of energy loss, which is mainly lost in the form of heat energy, and the electrolysis of electrolyte water will lead to changes in electrolyte concentration. Therefore, proper maintenance of the battery and timely replenishment of the water lost by evaporation are important measures to ensure the normal operation of the battery and extend the service life.
In general, lead sulfate batteries work by converting chemical energy into electrical energy for discharge through a chemical reaction that occurs between the positive and negative electrodes, and forcing a reverse reaction to charge by applying an applied voltage. Through the clear analysis of its structural composition, discharge and charging process, it can help us better understand and use this classic battery type.